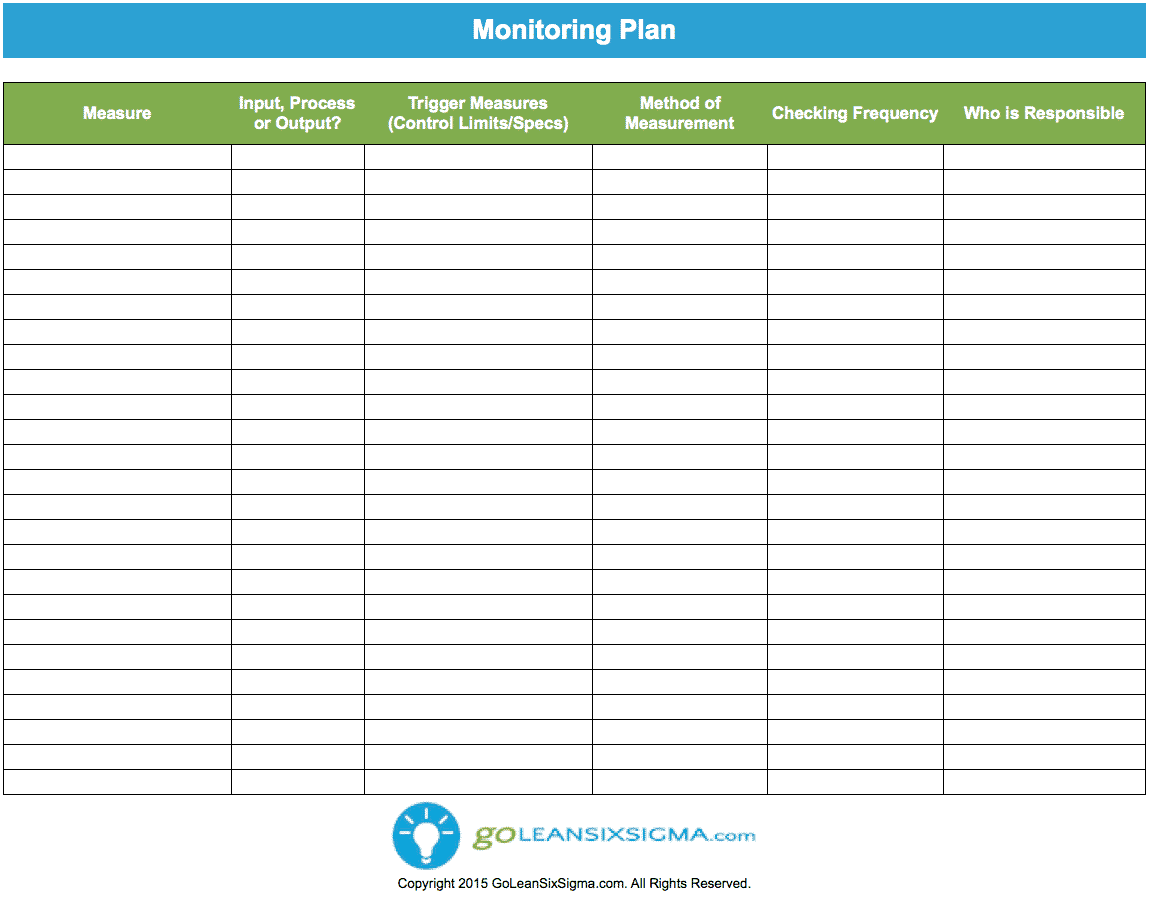
Web this clinical monitoring plan (cmp) establishes the guidelines for conducting monitoring visits and related tasks for monitoring murdoch children’s research institute (mcri). Web this template has been developed by the niams to assist the pi and the study team with the writing of a dsmp and details the elements to be included such as study overview,. Web investigators should consider using this template when developing the data and safety monitoring plan (dsmp) for clinical studies funded by the national institute of arthritis. Web instructions this template is a suggested format for a monitoring plan developed by tb survey teams. Guidance document that provides detailed descriptions of the nidcr clinical monitoring processes.
Ad learn how philips patient monitoring can help unlock capabilities across your system. Web guidance document that provides detailed descriptions of the nidcr clinical monitoring processes. Guidance document that provides detailed descriptions of the nidcr clinical monitoring processes. Web nidcr clinical monitoring guidelines: Web nidcr clinical monitoring guidelines.
Web ðï ࡱ á> þÿ ¯ þÿÿÿ«¬. Web clinical study report template. Web instructions this template is a suggested format for a monitoring plan developed by tb survey teams. Find out how our patient monitoring products can help deliver operational efficiencies. Web this clinical monitoring plan (cmp) establishes the guidelines for conducting monitoring visits and related tasks for monitoring murdoch children’s research institute (mcri).
Web ðï ࡱ á> þÿ ó ö. Find out how our patient monitoring products can help deliver operational efficiencies. Web using the data and safety monitoring plan (dsmp) template provided by the irb, or developed using an outline format or narrative summary as preferred by the investigator. Web nidcr clinical monitoring guidelines: Web nidcr clinical monitoring guidelines. Web instructions this template is a suggested format for a monitoring plan developed by tb survey teams. This guidance was created to aid investigators developing a data and safety monitoring plan (dsmp) to ensure the safety of research. Enhance clinical productivity, reduce procedure delays, and improve the patient experience Web the national institute of mental health (nimh) has developed the following guidance for investigators developing a data and safety monitoring plan (dsmp) to. Guidance document that provides detailed descriptions of the nidcr clinical monitoring processes. Web investigators should consider using this template when developing the data and safety monitoring plan (dsmp) for clinical studies funded by the national institute of arthritis. Web clinical monitoring plan template guidance for clinical research associates responsible for preparing a clinical monitoring plan download the guidance. Throughout the template there are suggested. Web guidance document that provides detailed descriptions of the nidcr clinical monitoring processes. Learn about the adt® medical health alert product line.
![Monitoring+Plan[1] Clinical Trial Pharmacy](https://i2.wp.com/imgv2-2-f.scribdassets.com/img/document/37906565/original/ce5fdac205/1585408870?v=1)






